Endoscopic assessment of sinonasal mucormycosis with SPIES: The ‘battlefield’ sign
VIOREL ZAINEA1,2, IRINA GABRIELA IONITA1,2, SILVIU PITURU1, CĂTĂLINA PIETROȘANU1,2, ANDREEA RUSESCU1, CRISTIAN DRAGOS STEFANESCU1,2, FLORENTINA GHERGHICEANU1, FLORIN ANGHELINA3,4, DRAGOS PALADE5,6 and RAZVAN HAINAROSIE1,2
1Department of ENT, ‘Carol Davila’ University of Medicine and Pharmacy, 050474 Bucharest; 2Department of ENT, ‘Prof. Dr. D. Hociota’ Institute of Phonoaudiology and Functional ENT Surgery, 061344 Bucharest; 3Department of ENT,
University of Medicine and Pharmacy of Craiova, 200349 Craiova; 4Department of ENT, Emergency County Hospital Craiova, 200642 Craiova; 5Department of ENT, ‘Grigore T. Popa’ University of Medicine and Pharmacy, 700115 Iasi;
6Department of ENT, ‘Sf. Spiridon’ Emergency University Hospital, 700111 Iasi, Romania Received July 16, 2021; Accepted August 17, 2021
DOI: 10.3892/etm.2021.11000
Abstract. Sinonasal mucormycosis is an extremely challenging pathology for the ear, nose and throat (ENT) surgeon from a therapeutic point of view. The disease affects immunocompromised patients and exhibits lethal potential. Although the diagnosis is relatively easy to suspect due to the distinctive clinical aspects which consists of black crusting present in the nasal fossae able to be confirmed by biopsy, the treatment requires resection of all affected tissue with safety limits. Due to the tendency of invasion associated with this fungal infection and taking into account the location involved (the sinonasal area) and the grave condition of these patients, it is extremely important to perform only the minimal resection necessary, but that includes all tissue infected by the fungus. This article presents a minimally invasive method of evaluation that can be performed during endoscopic surgical intervention and that aids the surgeon to better evaluate the affected area. It is associated with no additional risks for the patients, but it helps the surgeon to perform the intervention efficiently while not damaging healthy tissue. The authors consider that the method presented will aid the surgeon during the endoscopic surgical intervention in evaluating the lesion and resecting all the affected tissue while preserving healthy areas.
1.Introduction
Sinonasal mucormycosis represents an invasive, extremely serious fungal infection located in the nasal fossae and sinuses, with an evolutiontowards extension to nearby structures and with potential destructive capacity (1). Its incidence is increasing in immunosuppressed patients (2), but it can rarely be observed in patients with a competent immune system (3). The invasive infection has a rapid onset, and early diagnosisand treatment can be a ‘life savior’.
More than 2 million people suffer from diabetes mellitus in Romania, and the patient numbers are increasing. Sinonasal infection with mucormycosis is one of the most feared complications in patients with diabetes mellitus. The fungal agent can invade the blood vessels and will embolize, which can in turn lead to distant organs being infected. Usually, the diagnosis of mucormycosis is not immediately confirmed,and the specific treatment is commonly delayed. In invasive infection with mucormycosis, the treatment of choice is tissue debridement until reaching healthy tissue and administration of amphotericin B (4,5). If amphotericin B is not available, treatment can be initiated with posaconazole (6,7).
The rate of fatality for patients with mucormycosis is extremely high, from 30% to as high as 80% (8), even when the surgical treatment consisting of accurate debridement of the fungus and medical treatment with amphotericin B or with posaconazole are utilized (9). Renal failure and hemodialysis are also redundant complications of these substances which should be emphasized. Although the diagnosis is definitely suggested by the specific clinical and endoscopic evaluation due to the presence of black crusting, it must be confirmed by biopsy. The time factor is also extremely important due to the tendency of rapid spread that characterizes this disease. The management of these patients represents a team challenge, as it requires a multidisciplinary team that must treat the associated diseases that have contributed to the immunocompromised status that led to the onset of the fungal disease and the contribution of the ENT surgeon, who must resect all necrotic tissue while preserving as much of the regional anatomy as possible (10).
For the surgeon, it is crucial to see the boundaries between necrosis, the infected tissue, and the healthy tissue. Direct sinonasal assessment includes five stages: i) White light examination (without magnification); ii) microscopic assessment (light with magnification); iii) endoscopic assessment (light with magnification and mobility of the endoscopic image, also giving details using angulated optic versions with 0˚, 30˚, 70˚); iv) refined endoscopic assessment consisting of the following: a) with focus 0 mm meaning video contact endoscopy including deep magnification with x60 or x150); b) selection of wavelength for veins and capillary during narrow band imaging (NBI); c) optic filters (selected from soft), from SPIES (Storz Professional Image Enhancement System); d)autofluorescence of the normal and pathologic tissue; e) video contact endoscopy with SPIES (synchronous); f) video contact endoscopy with NBI; v) endoscopic 3D imaging.
Endoscopic surgery has forced the revision of the anatomic and surgical landmarks, has revised the notions of topographic anatomy and has proposed the use of these concepts in surgical dissection and risk assessment during dissection under the endoscope.
SPIES filters were developed by The Karl Storz Company and can be applied directly during endoscopic surgical interventions. We observed the surgical field using Standard, CLARA, CHROMA, CLARA + CHROMA association, SPECTRA A and SPECTRA B.
SPIES consists of Storz Professional Image Enhancement filters and these can be used on FULL HD camera and 3D cable as well. Applying these filters allows the surgeon to better define the boundaries of any lesion, be it a tumor or in the case of mucormycosis, the necrotic tissue and, more importantly the limit between infected and healthy tissue.
The CLARA filter aids the surgeon by increasing the light in dark places of the endoscopic field. CHROMA increases the contrast of the surgical field. SPECTRA A removes the red color, and the surgeon is able to obtain an image similar to the NBI image. SPECTRA B adds a 15% red to the SPECTRA A filter and the surgeon is able to better visualize the darkened area of the surgical field.
2.Materials and methods
The aim of the present article was to reveal the endoscopic assessment protocol for patients with sinonasal mucormycosis, as implemented in our clinics.
The objective was to emphasize during endoscopic surgical assessment and dissection a sign (or more) that may be characteristic for the histopathology of invasive fungal rhinosinusitis ‘in vivo and in situ’. These characteristic aspects during endoscopic surgery may help the surgeon to better define the area and lesions, to dissect in a more accurate and careful manner and to follow the profile of the lesion until the limit of the surrounding healthy tissue is reached. Reading the lesion in real‑time during endoscopic dissection helps the surgeon to accurately perform all the steps of the surgical intervention.
At the same time, assessing the endoscopic aspect of the lesion and defining the sinonasal area affected, the surgeon can be efficient even when an extemporaneous examination is not possible to be carried out in every step of the intervention. Thus, reading the lesion ‘in vivo and in situ’, the entire area affected and the surrounding regions of the lesion can be thoroughly examined, and the endoscopic intervention can be carried out under safe conditions, allowing, at the same time, for all tissues presenting with fungal invasion to be excised.
According to our previous observations, the main objec‑ tive of this study was to identify characteristic aspects of the affected tissue by mucormycosis and to define these aspects. The method consisted of sinonasal endoscopic assessment with a special endoscopic technology (SPIES) based on software filters, used in patients with invasive sinonasal mucormycosis. We wish to underline that patients that presented to our clinic with mucormycosis presented with important comorbidities and important immunodeficiency, most often due to poorly controlled diabetesmellitus.
The anesthetic and surgical assessment (ASA) are always difficult and sometimes dramatic because of the powerful inflammatory and infectious complications surrounding the lesion induced by the invasive fungus.
The results according to the research hypothesis are the identification of a characteristic endoscopic design (image) visible during the endoscopic surgical assessment of the sinonasal area affected by this invasive type of fungus.
The final result was similar to our expectations and was based on observations during the assessment of multiple cases of invasive fungalsinusitis in immunosuppressed patients that were referred to our clinics, patients that presented with associated various comorbidities, such as diabetes mellitus, neutropenia or a history of organ transplant. We attempted to make a comparison between the endoscopic image projected as a landscape and a ‘battlefield’.
The two enemies (mucormycosis and the yet remaining healthy tissue) are separated by a neutral area (a pale territory) (Fig. 1). This ‘battlefield’ sign evokes the 3 lines of a ‘battlefield’ as follows: 1) Dark green mixed with black color (mucormycosis and tissue necrosis) which defines the mucormycosis area; 2) a pale‑colored area (tissue ischemia which is undergoing necrosis) is to be noted surrounding the area described above (point 1). It can be defined as a ‘neutral field of the battle’; and 3) a pale pink area with enforced vascular design (the advancing front of the surrounding healthy and reactive tissue) consisting of an inflammatory reaction with a protective purpose.
Such an endoscopic surgical image with the 3 ‘battlefield’ areas is not so difficult to observe during the operation if the technologic endoscopic system based on filters is mainly used in Chroma and Spectra B modes (Fig. 2). These images show the presence of invasive sinonasal fungus, the area of attachment, the progression front of the lesions, the reaction of the surrounding healthy tissue, the intermediate area between the two ‘enemies’ (mucormycosis and healthy tissue). All of these landmarks help the surgeon to establish the strategy and make possible the procedure of debridement of the necrotic tissue up to the point of safe margins.
In this case, safe margins define exclusively healthy tissue (mucosal line, clear bone and total macroscopic removal of the sinonasal mucormycosis). Thus, the term ‘safe margins’ is used here similar to oncologic surgery principles.
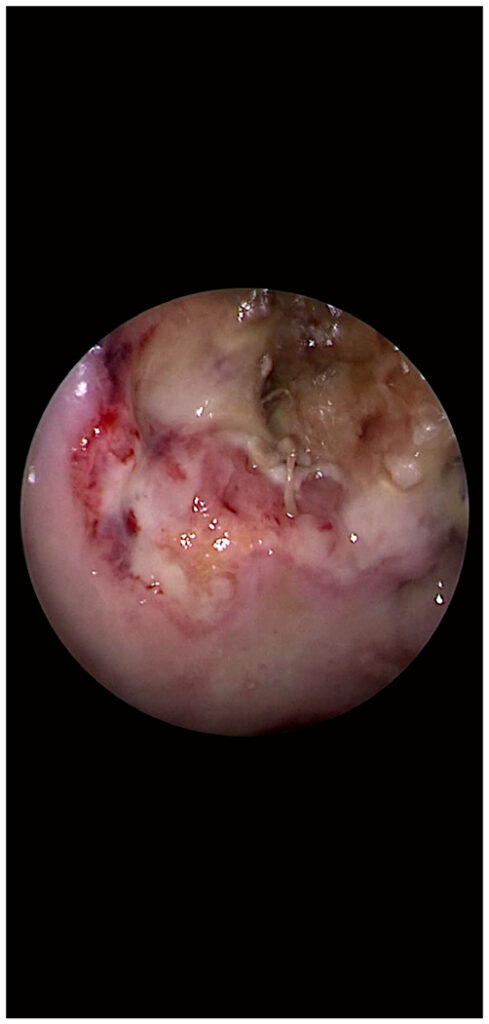
Figure 1. Endoscopic image of a patient with mucormycosis; white light endoscopy.
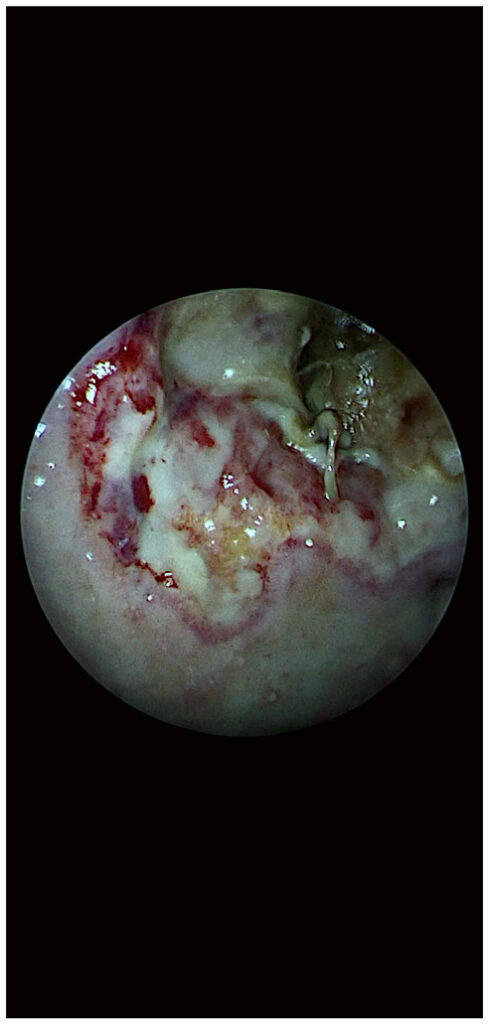
Figure 2. Endoscopic image of a patient with mucormycosis; SPIES endos‑ copy with SPECTRA B filter. SPIES, Storz Professional Image Enhancement System.
We tried to select suggestive endoscopic filtered images obtained during endoscopic surgery performed for patients with invasive mucormycosis which underline the ‘battlefield’ sign. These images and the boundaries defined with their help were later compared with images from the histopathological examination.
We consider that the best filters to be utilized to assess the ‘battlefield’ sign in mucormycosis infection are CLARA + CHROMA and SPECTRA B. Using only the CLARA filter will provide the surgeon with an increased luminosity of the surgical field, but the boundaries will not be well visual‑ ized. Using the SPECTRA A filter will darken the image too profoundly and the image will not be reliable.
3.Results
This paper aims to point out an endoscopic sign evidenced with the aid of SPIES technology during endoscopic surgical interventions performedfor patients with invasive fungal rhinosinusitis with mucormycosis.
The meaning of this endoscopic sign consists of the evidence of the three components of a ‘battlefield’: 1) Mucormycosis, an aggressive agent which is confirmed by evidence of mycologic infection. For a correct assessment and clear diagnosis computed tomography (CT), Magnetic Resonance Imaging (MRI) and sampling (biopsies) must also be performed; 2)neutral line which is tissue ischemia marking the start of the undergoing necrosis; and 3) reactive surrounding tissue.
The endoscopic surgeon must always look for this sign on the monitor during the progressing surgery, aiming to identify the border of the lesions, the neutral and reactive areas and finally to make a complete debridement and removal of the lesions induced by the fungal agent up to the healthy tissue (‘safe margins’).
The best filters to assess ‘safe margins’ include CLARA + CHROMA and SPECTRA B. Use of these filters allows the surgeon to better see the affected and healthy tissue. The SPIES technology is a valuable tool for assessing the limit between the healthy and the infected tissue. It is simple to apply during surgery and can be applied to any Hopkins type rigid endoscope with any angulation.
No matter how useful this method is in assuring a good outcome for the surgical intervention, the importance of the complex management of such patients must be emphasized. Although the complete removal of the necrotic tissue is essential for a positive evolution, these patients also require antifungal treatment and management of the underlying conditions that favored the infection with mucormycosis. One of the most frequently encountered systemic disease that is associated with mucormycosis is diabetes mellitus, patients that have trouble with maintaining adequate blood sugar levels (11). The long‑term imbalance of glycemia levels causes poor local defense mechanisms and a more difficult recovery, overall decreasing the survival chances of our patients (12). When evaluating a diabetic patient with a significant aggravation of the general status who is suspected of presenting with a fungal infection, we should consider the possibility of mucormycosis infection (13). A poor nutritional status may also lead to a significantly poor evolution (14). However, this is not the only condition that may be associated with mucormycosis. Patients with neoplasms, liver failure, neurologic disorders or hematological illnesses are at risk for opportunistic infections and may also present with such fungal infections (15). We must also keep in mind that patients must be made aware of the severity of the disease and the importance of their compliance to treatment, which may result in an overall increased survival (16).
Intensive care units will provide the best standard of care for patients with mucormycosis. Life support, systemic treatment and thorough monitoring are essential in the days following surgery. Only by maintaining such standards of care associated with the surgical intervention do such patients have a chance at recovery.
4.Discussion
During endoscopic interventions, currently surgeons have advanced endoscopic technology based on software using image filters which can better detect and point out the limits of any lesion, ranging from malignant tumors to invasive fungal rhinosinusitis (17).
This type of technology using selected filters, especially STANDARD, CLARA + CHROMA and SPECTRA B, can help the surgeon identify during surgery the so‑called ‘battle‑ field sign’ as presented above with photo documentation. The ‘battlefield’ signconsists in identifying on the endoscopic landscape (screen) the aforementioned 3 areas, each with its characteristic color, from darkgreen‑almost black to pale pink. The advantage of identifying this sign during endoscopic surgery for invasive fungal rhinosinusitis with mucormycosis is that it facilitates the progress of the steps of the operation until clear margins (safe margins) are reached as is targeted in oncologic surgery.
We always verify and compare the ‘battlefield’ sign with the specimens directly sampled from the tissue. It must be emphasized that the biopsy of the affected tissue still remains the gold standard of histologic diagnosis and it is always more accurate than the endoscopic examination,even with advanced SPIES filters (18).
The mycologic, CT and MRI examinations are always mandatory for a complete diagnosis (8). The management of these patients remains amultidisciplinary challenge (19), with the aim of the ENT surgeon to resect all infected tissue, while preserving as much of the normal anatomy as possible and to protect the vital areas, while treating all the underlying disease of the patient and administering the relevant antifungal treatment. This endoscopic method of evaluation will aid the ENT surgeon is assessing the lesion and performing a thorough excision, while preserving as much of the healthy tissue as possible in a narrow area with vital structures and adding no extra risks to these patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The information used and/or analyzed during the current study is available from the corresponding author on reasonable request.
Authors’ contributions
All authors (VZ, IGI, SP, CP, AR, CDS, FG, FA, DP and RH)
contributed to the acquisition of the data and critical revision
of the manuscript for intellectual content. VZ and CDS were responsible for the research design and manuscript drafting. CP and AR wereresponsible for the language editing. FG and FA were responsible for editing the article and images. DP and SP contributed to the literaturedata analysis and the critical interpretation and IGI was a major contributor in writing the manuscript. RH reviewed the manuscript. Allauthors read and approved the final version of the manuscript for publication.
Ethics approval and consent to participate
All patients have given their consent to participate in this study, and the study was conducted according to the principles of the Declaration ofHelsinki. Ethics committee approval was not necessary.
Patient consent for publication
All patients provided their consent for the publication of the data; the privacy data regulation was followed.
Competing interests
The authors declare no competing interests.
References
- Battikh R, Labbene I, Ben Abdelhafidh N, Bahri M, Jbali A, Louzir B, M’saddek F, Ferjani M, Gargouri S, Dhahri MA, et al: Rhinofacial mucormycosis:3 cases. Med Mal Infect 33: 427‑430, 2003.
- Soler ZM and Schlosser RJ: The role of fungi in diseases of the nose and sinuses. Am J Rhinol Allergy 26: 351‑358, 2012.
- Hussain S, Salahuddin N, Ahmad I, Salahuddin I and Jooma R: Rhinocerebral invasive mycosis: Occurrence in immunocompe‑ tent individuals. Eur JRadiol 20: 151‑155, 1995.
- Bouza E, Munoz P and Guinea J: Mucormycosis: An emerging disease? Clin Microbiol Infect 12: 7‑23, 2006.
- Brown J: Zygomycosis: An emerging fungal infection. Am J Health Syst Pharm 24: 2593‑2596, 2005.
- Rutar T and Cockerham KP: Periorbital zygomycosis (mucormy‑ cosis) treated with posaconazole. Am J Ophthalmol 142: 187‑188, 2006.
- Notheis G, Tarani L, Costantino F, Jansson A, Rosenecker J, Friederici D, Belohradsky BH, Reinhardt D, Seger V, Schweinitz DV and WintergerstU: Posaconazole for treatment of refractory invasive fungal disease. Mycoses 49 (Suppl 1): S37‑S41, 2006.
- Singh VP, Bansal C and Kaintura M: Sinonasal mucormycosis: A to Z. Indian J Otolaryngol Head Neck Surg 71 (Suppl 3): S1962‑S1971, 2019.
- Greenberg RN, Mullane K, Van Burik JA, Raad I, Abzug MJ, Anstead G, Herbrecht R, Langston A, Marr KA, Schiller G, et al: Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother 50: 126‑133, 2006.
- DeShazo RD, O’Brien M, Chapin K, Soto‑Aguilar M, Gardner L and Swain R: A new classification and diagnostic criteria for invasive fungal sinusitis.Arch Otolaryngol Head Neck Surg 123: 1181‑1188, 1997.
- Hainarosie R, Zainea V, Rusescu A, Iana RO, Ghindea T. Suceveanu AP, Stefanescu DC, Ionita IG, Pietrosanu C and Stoian AP: Management of infectious complications in diabetes mellitus mellitus patients. Romanian J Military Med 122: 46‑51, 2019.
- Ditu G, Voiculescu DC, Bodnarescu M, Pantea Stoian A, Hainarosie R, Stefan S and Serafinceanu C: Mucormycosis and Diabetes Mellitus.Proceedings of National ENT, Head and Neck Surgery Conference: Arad, Romania, June 06‑09, 2018, pp. 199‑204, 2018.
- Pantea Stoian A, Hainarosie R, Stoica R, Nitipir C, Paduraru DN, Andronache L, Oros M, Pituru S and Serafinceanu C: MOE‑A Severe Complication inDiabetes Mellitus. Proceedings of National ENT, Head and Neck Surgery Conference, Arad, Romania, June 6‑9, 2018, pp401‑405.
- Drăgoi CM, Moroșan E, Dumitrescu IB, Nicolae AC, Arsene AL, Drăgănescu D, Lupuliasa D, Ioniță AC, Pantea Stoian A, Nicolae C, et al: Insight into chrononutrition: The innermost interplay amongst nutrition, metabolism and the circadian clock, in the context of epigenetic reprogramming. Farmacia 67: 557‑571, 2019.
- Ioacara S, Tiu C, Panea C, Nicolae H, Sava E, Martin S and Fica S: Stroke mortality rates and trends in Romania, 1994‑2017. J Stroke CerebrovascDis 28: 104431, 2019.
- Țânțu MM, Stan GM, Rogozea L, Păunescu A, Pleșa CF, Nemeș RM, Nicolae C and Bisoc A: Drug use, a valid indicator for effective implementation of medical protocols. Revista de Chimie 70: 859‑862, 2019.
- Chandra RK, Conley DB and Kern RC: Evaluation of the endo‑ scope and endoscopic sinus surgery. Oolaryngol Clin North Am 42: 747‑52, 2009.
- Skiada A, Lanternier F, Groll AH, Pagano L, Zimmerli S, Herbrecht R, Lortholary O and Petrikkos GL; European Conference on Infections inLeukemia: Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conferenceon Infections in Leukemia (ECIL 3). Haematologica 98: 492‑504, 2013.
- Paleiwala SK, Zangeneh TT, Goldstein SA and Lemole GM: An aggressive multidisciplinary approach reduces mortality in rhinocerebralmucormycosis 7: 61, 2016.